2014: Adsorption properties of functionalized nanodiamonds in aqueous solutions of metal salts
Physicists from Lomonosov Moscow State Univ. (group of Senior Researcher, Tatiana Dolenko, Chair of Quantum Electronics and Associate Prof. Tatiana Laptinskaya) in collaboration with foreign scientists recently studied the adsorption properties of detonation nanodiamonds with respect to heavy metal and nitrate ions.
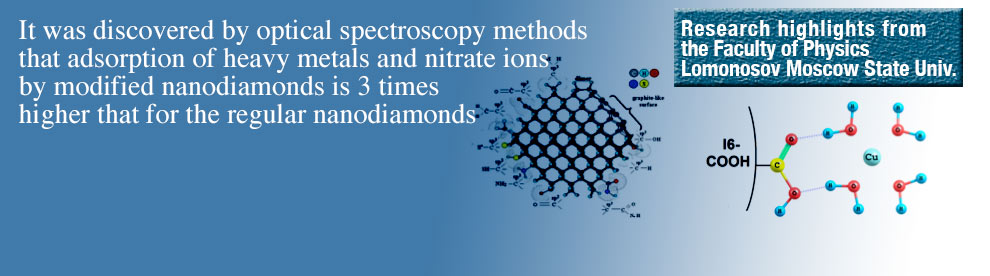
The adsorption properties of nanodiamonds (ND) I6 and I6COOH in aqueous solutions of nitrates of copper and lead were studied experimentally with the methods of correlation spectroscopy, absorption spectroscopy, Raman spectroscopy and infrared absorption. It was found that both NDs actively adsorb nitrate ions and metal cations, and the adsorption efficiency of I6COOH is about three times higher than that of I6 in respect to the ions. On the basis of the IR and Raman results, we propose a hypothesis about mechanisms of adsorption of nitrate ions and copper cations on the surface of the NDs, according to which the dominant role in the adsorption of Cu is played by physical adsorption and the dominant role in the adsorption of nitrate anions is played by chemical adsorption.
The results of this work have been published in the paper: T.A.Dolenko, S.A.Burikov, K.A.Laptinskiy, T.V.Laptinskaya, J.M.Rosenholm, A.A. Shiryaev, A.R.Sabirov, I.I.Vlasov. "Study of adsorption properties of functionalized nanodiamonds in aqueous solutions of metal salts using optical spectroscopy", J. of Alloys and Compounds 586, pp. S436–S439 (2014).